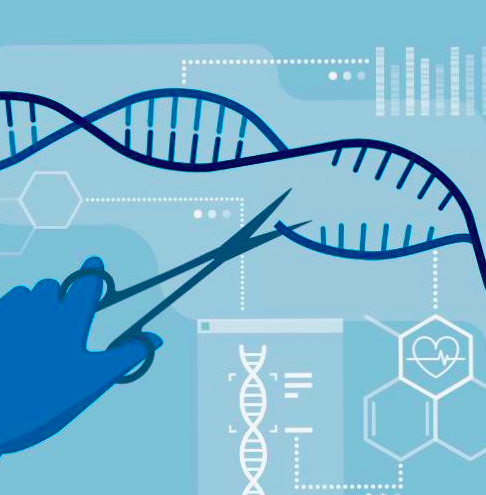
CRISPR-Cas9 – Genetic Scissors at Work
In 2020, the Nobel Prize in Chemistry was awarded to Jennifer A. Doudna and Emmanuel Charpentier for their development and understanding of the gene-editing tool, CRISPR-Cas9.
Charpentier has been quoted as saying this system is “mind-blowing.”
Protection at the molecular level
The CRISPR-Cas9 complex, while new to our own understanding, is not new to archaea or bacteria. The system we know has been present in these organisms for centuries, allowing them to create their own network of molecular defense.
Utilizing this bacterial line of defense, the possibilities are endless for humans.
How exactly does CRISPR-Cas9 work? In simple terms, CRISPR-Cas9 can be thought of as a pair of genetic scissors.
CRISPR is a series of short palindromic repeats, while Cas9 is the endonuclease that is guided by designed RNA specific to the experiment. CRISPR and Cas9 must work together as a system to allow for the process of gene editing.
By making a specific cut at either a targeted gene or region of DNA, this system then allows natural DNA processes to take over and repair the region, excising the area of issue and replacing it with either the correct sequence of DNA or another gene.
Here is the step-by-step process:
- Recognition – Guide RNA must be designed to target the region of interest, guiding the Cas9 “scissors” to the target
- Cleavage – Cas9 makes double stranded breaks, as DNA is a double-stranded molecule, to excise the region of interest
- Repair – Homology-directed repair and other host-cell machinery repair the excised region, either inserting another gene as directed by Cas9 or inputting the correct version of the sequence once there
Yes — this is a complicated process with plenty of scientific jargon that can make it confusing to those not familiar with the technique.
In summary, CRISPR-Cas9 can remove “bad” DNA and allow it to repair itself, opening up a world of possibilities for gene-editing in the future.
Designer babies: Seriously?
The movie “Gattaca” may not be that far out of reach…
Scientists hypothesize that with the power of CRISPR, certain diseases and disorders could be eradicated from a child before they’re even born. Some issues with this, of course, lie with the potential implications and damage that this gene-editing could cause. Is it worth the risk?
While parents may dream of getting to choose whether their child has blue eyes or red hair, this tool should be used for more pressing issues such as Tay-Sachs or phenylketonuria.
Most notably, scientist He Jiankui was under much scrutiny when he announced to the world that twin girls with edited genomes were to be born. Not only did this go against many within the scientific community, but he also risked human life and safety laws to practice this technique.
Human research is hard enough to get clearance for, as clinical trials must go through countless rounds of approval in order to use human subjects as opposed to other comparative species such as rats or monkeys. While scientists are unable to see the future impacts this research will have on the gene-edited children, they are consciously aware of the changes this could send spiraling in the scientific community.
Personalized treatment with targeted-gene therapy
With the ease of the CRISPR-Cas9 system, site-specific gene editing is within reach.
Traditional gene editing techniques rely heavily on viral vectors as delivery vehicles, which is the inactive form of a virus used as a means to deliver specific DNA sequences into cells and their genomes. Some issues with viral vectors are that they often are too large to integrate, can be inactivated by the cell’s own mechanisms and the requirement for cell division to spread the information throughout the body stalls the efficacy of this method.
This is where CRISPR-Cas9 comes in. While avoiding the need for unreliable viral vectors, this system uses molecular tools to directly make changes to the DNA sequence (genome) itself.
This technology has a future that is bright, from patient-specific tumor treatments to clinical trials for sickle-cell anemia.
What other clinical trials are expected to be progressing as CRISPR is pending FDA approval?
- Genetic blindness
- Diabetes
- Infectious diseases such as chronic UTIs and HIV/AIDS
- Inflammatory diseases
The ideas above are not the only areas of science that CRISPR is limited to. The list will continue to expand as the mechanism of action is better understood. To learn more, please reference the Innovative Genomics Institute.
Want to try your hand at using CRISPR-Cas9 in a lab setting? Dr. Song’s Advanced Methods Molecular Genetics class — split between undergraduate and graduate students — is an 8-week course covering topics such as genetic analysis of C.elegans (nematode) strains using CRISPR-Cas9 and homology-directed repair. Databases and applications are used to acquire genetic data and design individual student’s genetic engineering projects.
If you are interested in taking this course, please refer to the academic catalog number BIO 4255.